In recent years, the healthcare industry has undergone a digital transformation, with medical devices playing a pivotal role in patient care and treatment. These devices, ranging from insulin pumps and pacemakers to infusion pumps and MRI machines, have become integral to modern medicine. However, this increasing reliance on medical devices has also exposed vulnerabilities that can be exploited by cybercriminals, potentially endangering patients’ lives and compromising the integrity of healthcare systems. This article explores the importance of securing medical devices and provides an overview of strategies and best practices to ensure their safety and confidentiality.
The Growing Significance of Medical Device Security
The Pervasive Nature of Medical Devices
Medical devices are ubiquitous in healthcare settings, encompassing a wide range of technologies and functions. From monitoring vital signs to delivering precise doses of medication, these devices are essential for diagnosing and treating patients. With the advent of the Internet of Things (IoT) and the integration of these devices into larger healthcare systems, their security is of paramount importance.
The Threat Landscape
The increasing connectivity of medical devices exposes them to a myriad of cybersecurity threats. These devices are vulnerable to unauthorized access, data breaches, and even potential manipulation of their functionality. Malicious actors can exploit these vulnerabilities to gain access to patient data or, in some cases, directly harm patients by tampering with device settings.
Securing Medical Devices: Best Practices
Risk Assessment
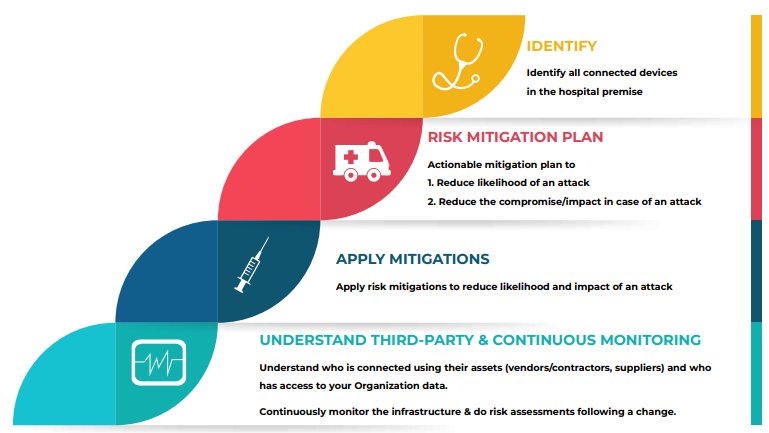
A thorough risk assessment is the first step in securing medical devices. Healthcare organizations must identify and evaluate the potential risks associated with each device. This includes assessing the device’s intended use, data handling capabilities, and potential impact on patient safety. A risk assessment should also consider the device’s exposure to network vulnerabilities and the level of protection required.
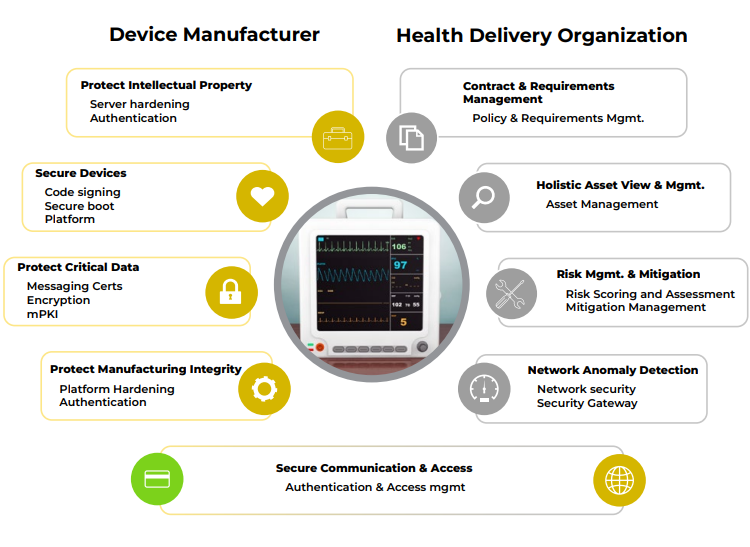
Secure Design and Development
Manufacturers should adopt secure design principles and adhere to cybersecurity best practices during the development of medical devices. This includes implementing encryption protocols, access controls, and regular software updates to patch vulnerabilities. Additionally, manufacturers should consider the entire lifecycle of the device, from design to disposal, and plan for secure end-of-life procedures.
Network Segmentation
Healthcare organizations should implement network segmentation to isolate medical devices from the broader hospital network. This limits the potential attack surface and ensures that even if one device is compromised, the entire network is not jeopardized. Segmentation should be complemented by strict access controls and monitoring.
User Authentication and Access Control
Robust user authentication mechanisms, such as biometrics or two-factor authentication, should be enforced for accessing medical devices. Access control policies should restrict device access to authorized personnel only, reducing the risk of unauthorized tampering.
Regular Updates and Patch Management
Software vulnerabilities are a common entry point for cyberattacks. Regularly updating and patching both the operating systems and software of medical devices is essential to mitigate these risks. Manufacturers should provide a mechanism for easy and secure updates, and healthcare organizations must prioritize timely installation.
Intrusion Detection and Monitoring
Implementing intrusion detection systems and continuous monitoring of medical devices and their network connections can help identify suspicious activities in real-time. This proactive approach allows for rapid response to potential security incidents.
Incident Response Plan
Having a well-defined incident response plan is crucial for mitigating the impact of security breaches. Healthcare organizations should establish clear protocols for reporting and responding to security incidents involving medical devices. This plan should encompass communication, isolation of compromised devices, forensic analysis, and patient notification if necessary.
Training and Awareness
Healthcare staff should receive regular training on cybersecurity best practices and be aware of the potential risks associated with medical devices. Creating a culture of security awareness among employees can significantly reduce the likelihood of human error leading to security breaches.
Leading Medical device security tools
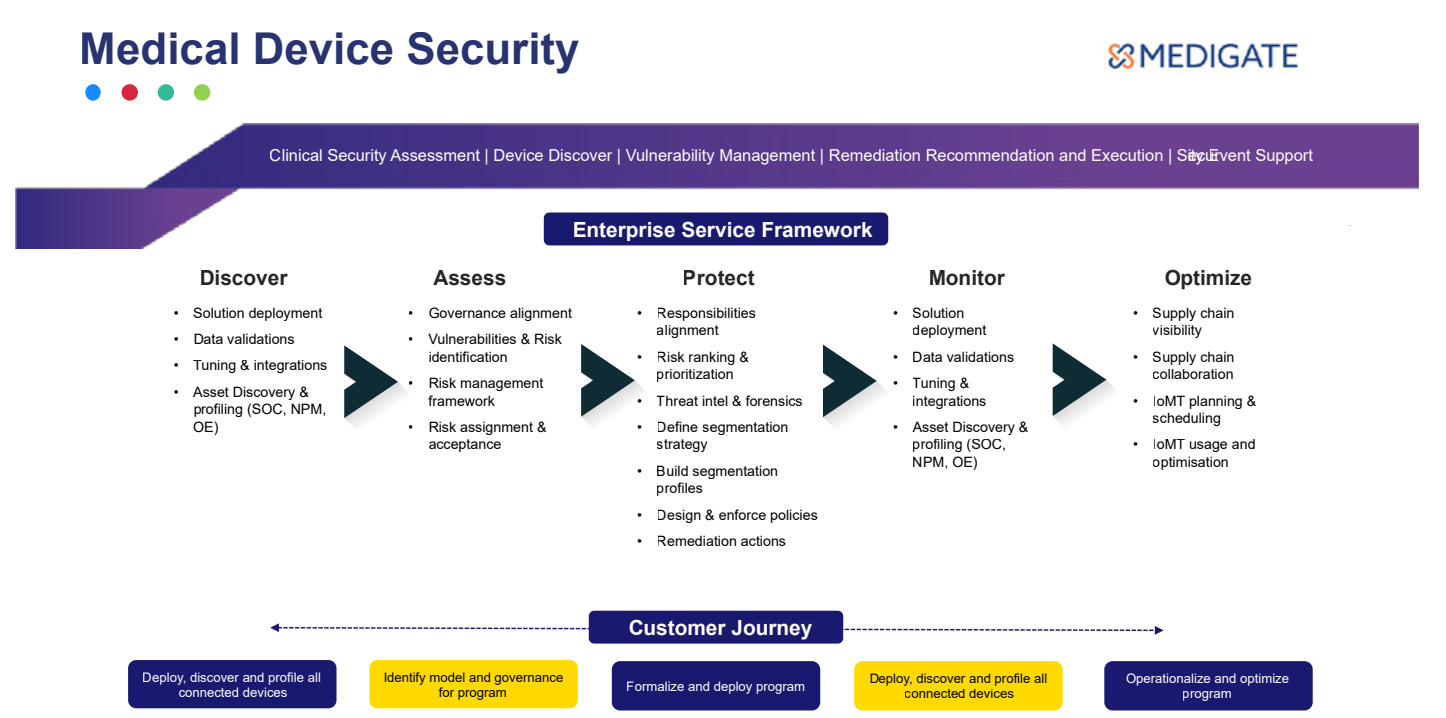
- Medigate
- Asimily
- ORDR
Securing medical devices is not just a matter of compliance; it is a critical component of patient safety and data protection in healthcare. As medical technology continues to advance, the need for robust cybersecurity measures becomes increasingly evident. By conducting thorough risk assessments, implementing secure design practices, and maintaining a proactive security posture, healthcare organizations can ensure that medical devices fulfill their intended purpose without compromising patient well-being or data integrity. In this digital age, securing medical devices is an ethical and practical imperative that should not be overlooked.

Author
Karthikeyan Manoharan
Karthikeyan Manoharan is an accomplished Cybersecurity Architect with over 19 years of experience in the field. Karthik has successfully delivered several Cybersecurity projects viz Security Operations Center implementation, and compliance-related programs for reputed organizations across the Globe. Apart from Cybersecurity, he is a numismatist and interested in learning about several cultures.